
Top Performing Drug – Tagrisso (October Edition)
Shots:
- In continuation of our previous series on the Top-Performing Drug of the month, based on 2021 revenue, this month we have selected Tagrisso and prepared a curated analysis report for our readers
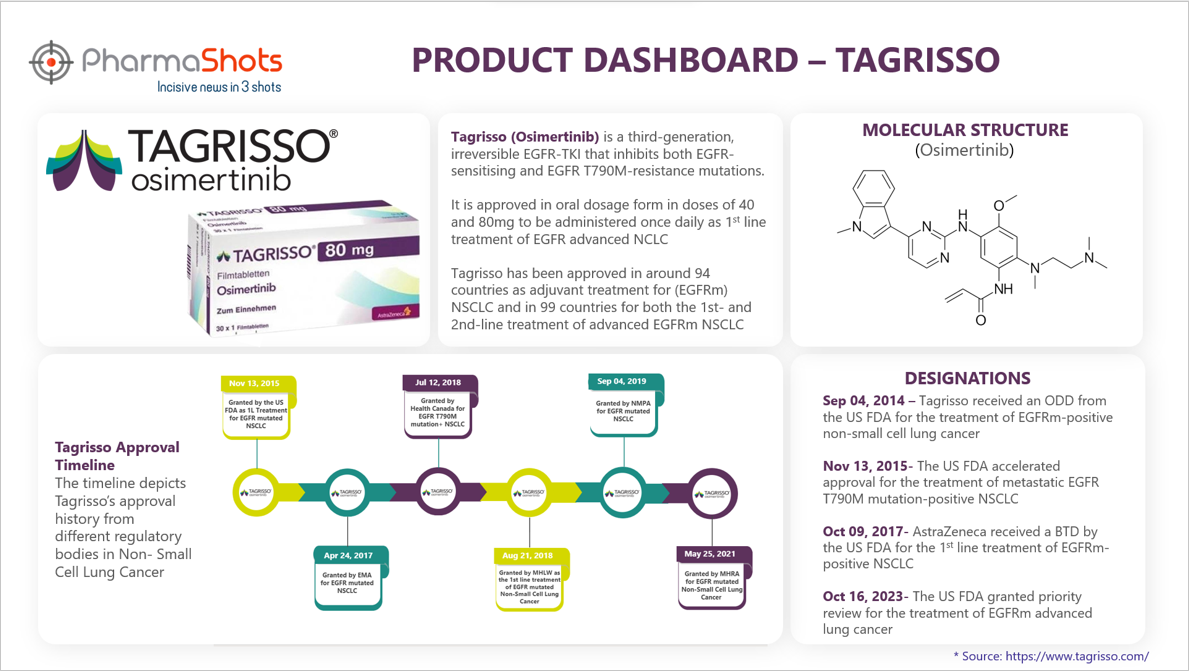
- Tagrisso is indicated for the treatment of non-small-cell lung carcinomas with specific mutations. It is a 3rd generation drug that belongs to the class of EGFR tyrosine kinase inhibitor
- PharmaShots presents a concise take on the key features of Tagrisso with a detailed analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis and informative KOL reviews
Active Ingredient: Osimertinib
Dosage Forms & Strengths: Tablets: 80 mg and 40 mg
Mechanism of Action: Epidermal growth factor receptor antagonists
Originator: AstraZeneca
First Approvals: The table below depicts the first approvals of Tagrisso from different regulatory agencies.
.png)
Revenue Analysis1
With approvals across 94 countries for the adjuvant treatment of patients with early-stage EGFR mutated (EGFRm) Non-Small Cell Lung Cancer (NSCLC) and approvals across 99 countries as 1st and 2nd-line treatment of advanced EGFRm NSCLC, Tagriso saw a massive upsurge in its total sales since 2018. The highest percentage change seen in the product’s total revenue was in 2020, with net sales of $4.33B as compared to $1.47B generated in 2019. This 194.62% boost in revenue was attributed to the strong penetration of Tagrisso across all markets.
The following graph illustrates the revenue analysis for the last five years' sales of Tagrisso.
.png)
Approved Indications2:
Tagrisso is approved as:
- An adjuvant therapy for adult patients with non-small cell lung cancer (NSCLC) who have undergone tumor resection and possess EGFR exon 19 deletions or exon 21 L858R mutations
- 1L treatment for adult patients with metastatic NSCLC carrying EGFR exon 19 deletions or exon 21 L858R mutations
- Adult patients with metastatic EGFR T790M mutation-positive NSCLC whose disease have progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy
Clinical Trials Analysis3
Clinical trial analysis is vital for advancing medical science, improving patient care, and making informed decisions about the safety and efficacy of new treatments. It underpins the foundation of evidence-based medicine and regulatory approval processes, leading to better healthcare outcomes and the development of innovative therapies.
The following dashboard illustrates the clinical trials associated with Tagrisso.
.png)
*Active trials include Recruiting; Active, Not Recruiting; Enrolling by Invitation, and suspended
*Inactive trials include Terminated; Withdrawn; Unknown Status
*Planned trials include Not, yet recruiting
Tagrisso Trials Representation (Country-wise)3
Below graphs depicts ongoing trials investing Tagrisso.
.png)
Product Dashboard
PharmaShots presents an illustrative infographic, highlighting essential metrics and pertinent information about Tagrisso.
Tagrisso Alternative Drugs4
In response to Tagrisso, several althernative drugs are available in the market to treat different indications. Some of the substitute drug for Tagrisso include:
.png)
Tagrisso Pipeline Analysis3
PharmaShots presents an extensive analysis of Tagrisso’s pipeline, including the ongoing Phase II and Phase III studies for various indications. The table below depicts an overview of these studies.
.png)
Tagrisso SWOT Analysis2, 3, 7, 8, 9, 10, 11
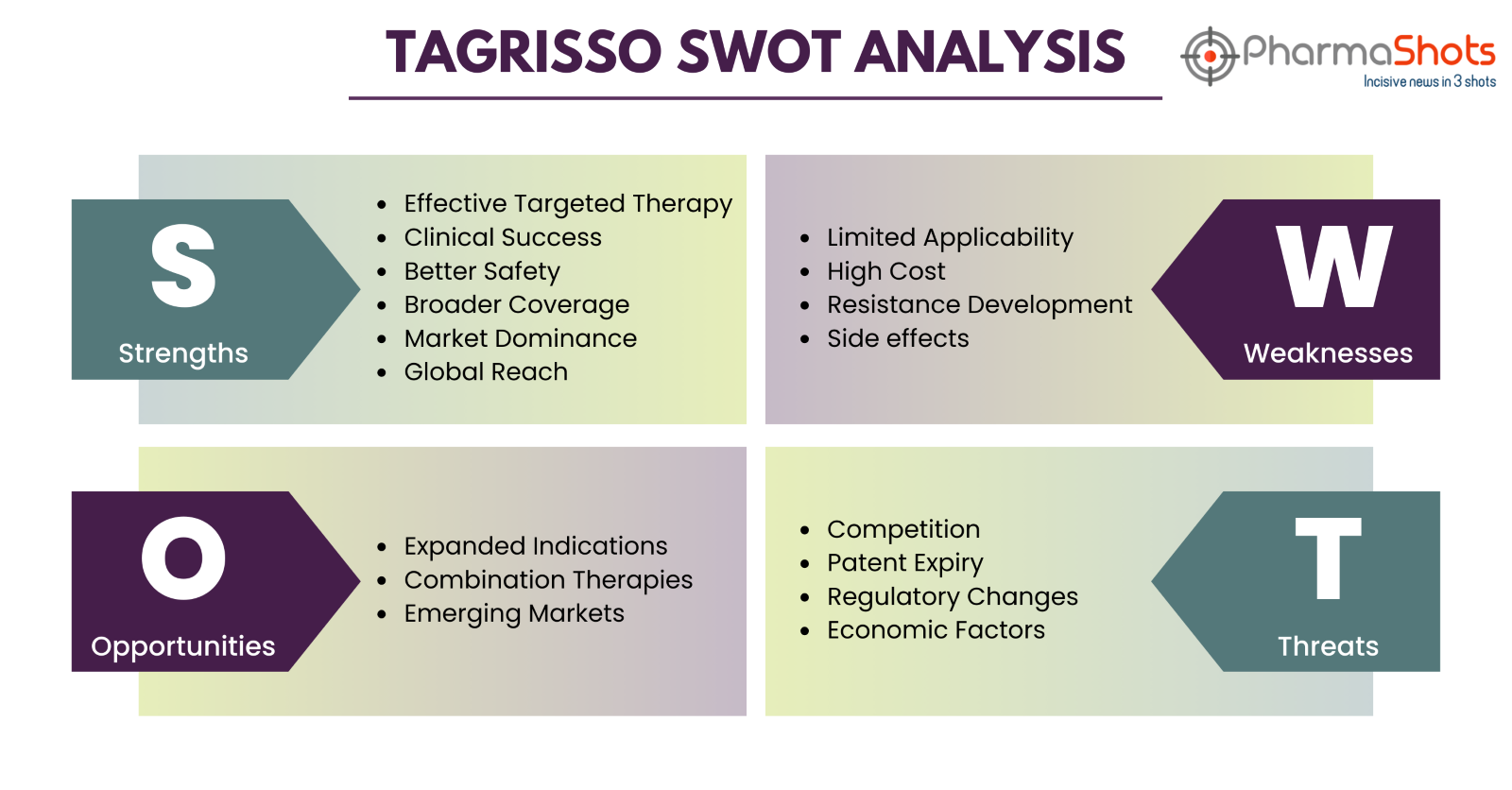
Strength
- Effective Targeted Therapy: Tagrisso is a leading targeted therapy for non-small cell lung cancer (NSCLC) with EGFR mutations, providing a more focused and effective treatment option1
- Clinical Success: Tagrisso has demonstrated significant clinical success, improving PFS in clinical trials. It is a highly effective treatment for EGFR-mutated NSCLC, with mPFS of 10.9 mos. in the 1L setting and 6.8 mos. in the 2L setting1, 4
- Better Safety: Tagrisso is well-tolerated, with a safety profile that is comparable to other EGFR tyrosine kinase inhibitors (TKIs)2
- Broader Coverage: Tagrisso has a broad label, covering first-, second-, and third-line treatment of EGFR-mutated NSCLC2
- Market Dominance: It holds a dominant position in the market for targeted therapies against EGFR-mutated NSCLC3
- Global Reach: AstraZeneca has a strong global presence and distribution network, ensuring wide availability, especially in Oncology
Weakness
- Limited Applicability: Tagrisso is only effective for patients with specific EGFR mutations, limiting its use to a subset of NSCLC patients6
- High Cost: The high cost of Tagrisso can be a barrier to accessibility for some patients and healthcare systems. With a wholesale acquisition cost of $13,771 per month7
- Resistance Development: Some patients may develop resistance to Tagrisso over time, limiting its long-term effectiveness5
- Side Effects: Like many cancer treatments, Tagrisso can have side effects, including diarrhea, skin problems, and interstitial lung disease4
Opportunity
- Expanded Indications: Research is ongoing to expand the use of Tagrisso to other cancer types or mutations, potentially increasing its patient base8
- Combination Therapies: Tagrisso may be used in combination with other drugs to enhance its effectiveness in treating different tumors8
- Emerging Markets: Entering emerging markets with unmet medical needs can drive growth for Tagrisso
Threats
- Competition: Rival drugs in development or currently on the market could challenge Tagrisso's market share, such as gefitinib/erlotinib in class and others outside class as well.
- Patent Expiry: The drug's patent protection may expire, allowing the entry of generic alternatives, which can reduce pricing and market share
- Regulatory Changes: Evolving regulations and policies may impact drug approval, pricing, and market access
- Economic Factors: Economic downturns can affect patient access to expensive medications like Tagrisso
Patient Stories5
Patients' stories are the key resources as they provide a holistic perspective on the impact of a medication on individuals' lives, improve healthcare decision-making, and contribute to the overall understanding of the drug's efficacy and safety in real-world settings. We have summarized some of the patients’ stories for Tagrisso:
- PETER’S STORY: Peter began to develop chest infections which were being treated by antibiotics. He underwent x-ray and was diagnosed with lung cancer. On discussion with the doctor, he received Osimertinib. He says, “Treatment comes with many side effects but I’m lucky. They haven’t impacted on how I live my life and Osimertinib makes my lung cancer stable, making living with lung cancer more manageable than I expected.”
- JEN’S STORY: Jen started to experience a lingering cough for which she was prescribed over-the-counter medications. After a few days, she underwent x-ray and was diagnosed with stage IV metastatic EGFR-sensitizing NSCLC. She received Tagrisso for 6 months and experienced a significant reduction in tumor volume and activity. She says, “I’ve been NED (no evidence of disease), thanks to Tagrisso, and to the willingness of my oncologist and surgeon to think outside the box.”
- GREG’S STORY: Greg was diagnosed with EGFR mutated stage IV lung cancer in 2004 and was enrolled in the clinical at Mass General Hospital Cancer Center. He received Tagrisso found improvement in his health condition. He says, “Healthwise, I've just switched from Iressa to Tagrisso, the targeted combination drug for EGFR and the T790 resistance gene. I've been in Tagrisso for a month and so far, things seem good. I've seen improvement in symptoms and have seen no side effects.”
KOL* Reviews6
KOL reviews offer valuable perspectives on different products and services. These reviews prove beneficial for consumers who engage in product research and prefer to read multiple reviews before making a purchase. Here are a few KOL reviews regarding Tagrisso
- Pasi A. Jänne, medical oncologist at Dana-Farber Cancer Institute said, “Patients received nearly nine additional months before their EGFR-mutated non-small cell lung cancer progressed as a result of the addition of chemotherapy to standard-of-care osimertinib, building on the strong efficacy we have already seen with osimertinib monotherapy. With these convincing data, patients may soon have a choice of two highly effective osimertinib-based treatment options in this advanced disease setting.”
- Masahiro Tsuboi, Director of Japan’s National Cancer Center Hospital East said, “The updated ADAURA results show us that adjuvant osimertinib not only continues to strikingly prolong the time patients with early-stage EGFR-mutated lung cancer are living cancer-free after surgery, but also continues to reduce the risk of tumors recurring in the central nervous system over time. These results reinforce adjuvant osimertinib as a standard-of-care treatment for these early-stage lung cancer patients who previously had no targeted treatment options after surgery, and who otherwise face high rates of disease recurrence.”
- Roy S. Herbst, Chief of Medical Oncology at Yale Cancer said, “Adjuvant TAGRISSO has demonstrated an unprecedented disease-free survival benefit for early-stage lung cancer patients with EGFR mutations who face high rates of recurrence even after successful surgery and subsequent chemotherapy. This approval reinforces how critical it is to test all lung cancer patients for EGFR mutations before deciding how to treat them and regardless of their stage at diagnosis. This will help ensure as many patients as possible can benefit from this potentially practice-changing treatment.”
- Susan Galbraith, AstraZeneca’s EVP, Oncology R&D said, “Tagrisso cut the risk of death by more than half in the adjuvant setting, further establishing this transformative medicine as the backbone treatment for EGFR-mutated lung cancer. These results emphasise the importance of diagnosing patients with lung cancer early, testing for EGFR mutations and treating all those with an EGFR mutation with Tagrisso.”
- Dave Fredrickson, EVP at Oncology Business Unit said, “For the first time, a targeted, biomarker-driven treatment option is available to patients in the US with early-stage EGFR-mutated lung cancer. This approval dispels the notion that treatment is over after surgery and chemotherapy, as the ADAURA results show that TAGRISSO can dramatically change the course of this disease. We remain committed to treating cancer patients earlier, when they may still have a chance of being cured.”
* Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL tracking services? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
- AstraZeneca Annual Report
- Tagrisso.com
- ClinicalTrials.gov
- Medical News Today
- Roycastle, Breathinghope and Massgeneral
- AstraZeneca PR and Businesswire
- The Lancet Oncology
- Cancer Therapy Advisor
- JAMA Network
- NIH
- NEJM.org
Related Post: https://pharmashots.com/16108/top-performing-drug-%E2%80%93-ozempic-september-edition
Tags

Shivani was a content writer at PharmaShots. She has a keen interest in recent innovations in the life sciences industry. She was covering news related to Product approvals, clinical trial results, and updates. We can be contacted at connect@pharmashots.com.














